
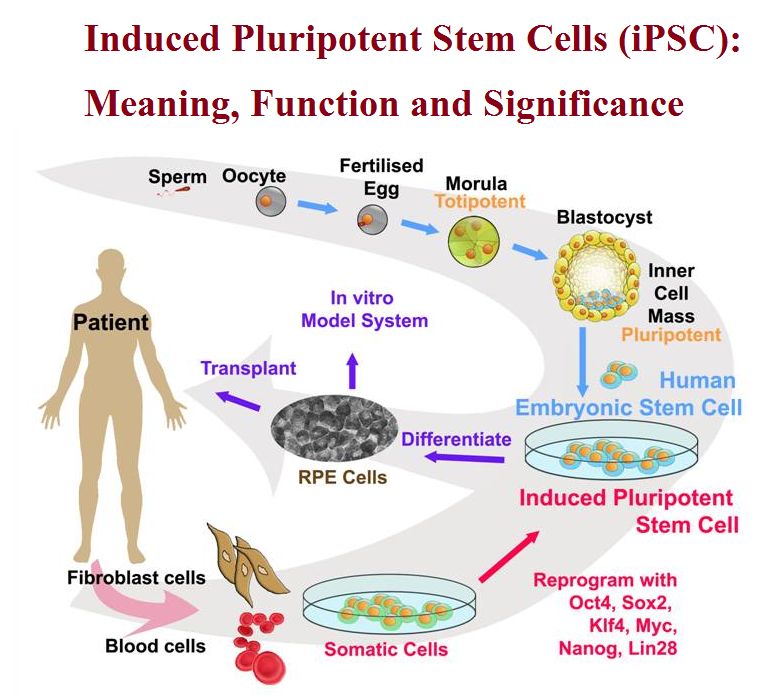
It has been theorized that certain epigenetic factors might actually work to clear the original somatic epigenetic marks in order to acquire the new epigenetic marks that are part of achieving a pluripotent state. Įpigenetic factors are also thought to be involved in the actual reprogramming of somatic cells in order to induce pluripotency. Some of the similarities between ESCs and iPSCs include pluripotency, morphology, self-renewal ability, a trait that implies that they can divide and replicate indefinitely, and gene expression. These induced cells exhibit similar traits to those of embryonic stem cells (ESCs) but do not require the use of embryos. This was then followed in 2007 by the successful induction of human iPSCs derived from human dermal fibroblasts using methods similar to those used for the induction of mouse cells. The ability to induce cells into a pluripotent state was initially pioneered in 2006 using mouse fibroblasts and four transcription factors, Oct4, Sox2, Klf4 and c- Myc this technique, called reprogramming, later earned Shinya Yamanaka and John Gurdon the Nobel Prize in Physiology or Medicine. These transcription factors play a key role in determining the state of these cells and also highlights the fact that these somatic cells do preserve the same genetic information as early embryonic cells. Induced pluripotent stem cells, commonly abbreviated as iPS cells or iPSCs, are a type of pluripotent stem cell artificially derived from a non-pluripotent cell, typically an adult somatic cell, by inducing a "forced" expression of certain genes and transcription factors. Main article: Induced pluripotent stem cells This pathway entails erasure of CpG methylation (5mC) in primordial germ cells via the initial conversion of 5mC to 5-hydroxymethylcytosine (5hmC), a reaction driven by high levels of the ten-eleven dioxygenase enzymes Reprogramming is facilitated by active DNA demethylation involving the DNA base excision repair enzymatic pathway. In mouse primordial germ cells, genome-wide reprogramming leading to totipotency involves erasure of epigenetic imprints. Work with zebrafish and mammals suggest a further interplay between miRNA and RNA-binding proteins (RBPs) in determining development differences. Research on Caenorhabditis elegans suggests that multiple mechanisms including RNA regulation may play a role in maintaining totipotency at different stages of development in some species. The inner cell mass, the source of embryonic stem cells, becomes pluripotent. Approximately four days after fertilization and after several cycles of cell division, these totipotent cells begin to specialize. After reaching a 16-cell stage, the totipotent cells of the morula differentiate into cells that will eventually become either the blastocyst's Inner cell mass or the outer trophoblasts. In the first hours after fertilization, this zygote divides into identical totipotent cells, which can later develop into any of the three germ layers of a human ( endoderm, mesoderm, or ectoderm), or into cells of the placenta ( cytotrophoblast or syncytiotrophoblast). Human development begins when a sperm fertilizes an egg and the resulting fertilized egg creates a single totipotent cell, a zygote. The human development model can be used to describe how totipotent cells arise.

Stem cells resembling totipotent blastomeres from 2-cell stage embryos can arise spontaneously in mouse embryonic stem cell cultures and also can be induced to arise more frequently in vitro through down-regulation of the chromatin assembly activity of CAF-1. In 2011, research revealed that cells may differentiate not into a fully totipotent cell, but instead into a "complex cellular variation" of totipotency. The conversion to totipotency is complex and not fully understood. Ī fully differentiated cell can return to a state of totipotency. In contrast, pluripotent cells can only differentiate into embryonic cells. In the spectrum of cell potency, totipotency represents the cell with the greatest differentiation potential, being able to differentiate into any embryonic cell, as well as any extraembryonic cell. Spores and zygotes are examples of totipotent cells. totipotentia, "ability for all ") is the ability of a single cell to divide and produce all of the differentiated cells in an organism. Only the morula's cells are totipotent, able to become all tissues and a placenta. These stem cells can become any tissue in the body, excluding a placenta. Pluripotent, embryonic stem cells originate as inner mass cells within a blastocyst.


 0 kommentar(er)
0 kommentar(er)
